Another medication, another chalky pill to swallow? Even getting your medically necessary prescriptions through a vein or an injection in the skin/muscle can have moderate side effects and inconveniences. The state-of-the-art in delivering the right amount of agent to the right place in the human body to treat or diagnose disease, known as targeted drug depot, is rapidly coming to a new level also through the exciting technology of hydrogels.
Moreover, another important approach to carry drugs to target is to link them to a specific molecule, an "antenna" who has particular affinity for the specific kind of cells we need to treat (as cancer cells), and can lead there the attached drug.
I have been working on the drug delivery topic since my Ph.D. thesis; for me it is a stimulating research, in which the physico-chemical knowledge about polymers and hydrogels can be used in the pharmaceutical field, making a positive union helpful for human health.
One of the approaches to obtain a controlled release of drugs in a specific place is to entrap them in hydrogels; the discovery of these innovative systems allow for the "smart" delivery of drugs and bioactive molecules, optimizing therapeutic effects and avoiding undesired side effects (such as poor absorption from oral intake, nausea, pain at injection site, inadequate targeting, etc.).
Hydrogels, in their simplest form, are obtained from natural and synthetic polymers and contain a high percentage of water, producing a semi-solid material that has tailored structural and mechanical properties. A well-known approach, hydrogels are able to produce a controlled release of a substance that is entrapped in the gel by the synthesis process.
Biopolymer-based hydrogels are specifically developed for living systems, such as those synthesized from polysaccharides (natural polymers such as sugars and starches) and proteins, so that the complex biological and chemical compatibility requirements are more likely to be met and the gel can be easily used in the human body.
The principle behind them is quite elegant -- basically, due to their high molecular weight, polysaccharides in water and in particular conditions (e.g. in presence of salts), form physical gels spontaneously, also in presence of drugs in solution that remain entrapped within the gel structure. These structures are classified as either physical or chemical gels according to the type of linkage present between the polymer chains and once synthesized as such, are suitable for a modified drug delivery. The release of the drug from the gel matrix is controlled by simple diffusion, and in physiological conditions, it is a slow release that can be measured.
Thus, one important application of such structures is the drug depot: surpassing the conventional ways of drug administration (oral, intravenous etc.), it is now feasible to deliver the drug directly in the specific site of action, in particular, in the case of local inflammation or solid tumours.
These drug depots are new hydrogels, specifically developed to be injectable in the presence of the drug and then to be cross-linkable inside the body. Given at the same time, drug depots achieve a local delivery and a prolonged release of the active molecule or protein in a non-invasive manner. For example, the Interpenetrating Polymer Network (IPN) gels, based on calcium alginate and chemically modified dextran, cross-linkable by ultraviolet light, were developed and tested in culture [Matricardi P. et al., Biomacromolecules, 2008, 9, p.2014-2020], as well as the hyaluronan (HA)-based "click-gels", which are cross-linkable after injection by a simple and fast chemical reaction ("click") [Crescenzi et al., Biomacromolecules, 2007, 8, p.1844-1850 and patent WO 2008031525, 2008-03-20].
Finally, I show you an example of active drug targeting, in particular for anticancer therapy. Among the polysaccharides, hyaluronan or HA -- an important molecule (glycosaminoglycan) found almost everywhere in the human body -- shows a selective interaction with a specific cellular receptor, the CD44, which is over-expressed in many cancers. By the linkage of an anticancer drug on the HA chain, a more selective target and release of the chemotherapeutic agent to tumour cells can be achieved [Di Meo C. et al., Macromolecular Bioscience 2008; 8; p. 670-681 and Biomacromolecules 2007; 8; p. 552-559; Platt V. M. et al., Molecular Pharmaceutics, 2008, 5, p. 474-486].
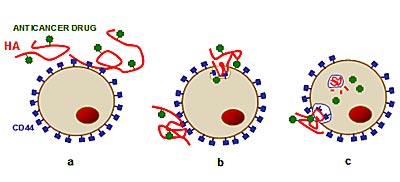
Illustration: Interaction of HA-drug with CD44 receptors on tumour cell (a), cell absorbs molecule by engulfing it through the CD44 "door" (b), HA-drug degrades and drug is released directly into the key areas of the cell (e.g. lysosomes), causing it to die (c).
(Chiara Di Meo, University of Rome - La Sapienza, www.atomiumculture.eu)